Q- A well-sealed room contains 80 kg of air at 200 kPa and 25°C. Now solar energy enters the room from windows at an average rate of 1 kJ/s while a 100-W fan is turned on to circulate the air in the room. If heat transfer through the walls is negligible, what will be the air temperature in the room …
Solution Search
. : "Heat and Thermodynamics"
Thermodynamics
Q- Air enters the compressor of a gas turbine plant (operating at steady state) at ambient conditions of 1 bar, 250C with low velocity and exits at 10 bar, 3500C with a velocity of 90 m/s. The compressor is cooled at a rate of 1500 kJ/min and the power input to the device is 250 kW. Determine the ma…
Thermodynamics: Heat Pump
Q- A heat pump is used to maintain a house at a constant temperature of 230C. The house is losing heat to the outside air through the walls and the windows at a rate of 60000 kJ/h and the energy generated within the house from lights and appliances amounts to 5000 kJ/h. For COP of 2.5, determine th…
Thermal properties
Q- At what speed an ice bullet (0oC) is to be fired on a target so that the whole ice in it is vaporized (100oC)? Consider the whole energy of bullet remains in it after hitting the target. (specific heat of water = 4.18 J/g/0C; Latent heat of ice = 334 J/g and latent heat of steam = 2272 J/g)
Thermodynamics
Q- A well-insulated rigid cylinder is divided in to 2 compartments by a conducting piston that is free to move freely. Initially one side of the piston contains 1 m3 of O2 at 5 bar, 800C and the other side contains 1m3 of N2 at 7 bar, 200C. The piston is released and the system is allowed to come …
Thermodynamics
Q- 15 kg of air in a piston cylinder device is heated inside the cylinder from 250C to 750C by passing current through a resistance heater inside the cylinder. The pressure inside the cylinder is kept constant at 2 bar during the process and the heat loss of 50 kJ occurs. Determine the electrical en…
Heat Radiation
Q- A student is trying to decide what to wear. His bedroom is at 20.0 degrees Celsius. His skin temperature is 35.0 degrees Celsius. The area of his exposed skin is 1.50 m2. People all over the world have skin that is dark in the infrared, with emissivity about 0.900. Find the net energy loss from h…
Thermal Physics
Q- Work is done on the water by a rotating paddle wheel, which is driven by two blocks falling at a constant speed. The temperature of the stirred water increases due to friction between the water and the paddles. If the energy lost in the bearings and through the walls is neglected, then the loss i…
Heat and Thermodynamics
Q- A spherical tank contains 1 mole of helium gas at 10 bar and 300 K. The gas is slowly released to the atmosphere in such a way the remaining gas is maintained at constant total energy U. Heating or cooling may be used to keep U constant during the venting. Helium behaves as an ideal gas with a co…
Conduction Of Heat
Q- A glass windowpane has an area of 3.00 m2 and a thickness of 0.600 cm. If the temperature difference between its faces is 25.0 degrees Celsius, what is the rate of energy transfer by conduction through the window? (coefficient of conductivity for glass to be taken as 0.8 W/(m.K)

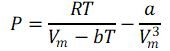 .
.
Comments